There are a total of 48 valence electrons in the Lewis structure for SF6. Note that Sulfur (S) is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur (S) atom. Step 1 – H2SO4 Valence Electrons. Oxygen and Sulfur atoms possess six electrons in their valence shells. Both of them come under the VIA group in the periodic table. By reading below you can check how we can calculate the total valence electrons in the molecule.
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. It has a chemical formula of SF2 and can be generated by the reaction of Sulphur Dioxide and Potassium Fluoride or Mercury Fluoride. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape.
| Name of molecule | Sulfur Difluoride ( SF2) |
| No of Valence Electrons in the molecule | 20 |
| Hybridization of SF2 | sp3 hybridization |
| Bond Angles | 98 degrees |
| Molecular Geometry of SF2 | Bent |
SF2 Valence electrons
For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride.
Total number of valence electrons for SF2 – Valence electrons of Sulphur + Valence electrons of Fluorine
Sulfur has six valence electrons in its outer shell.
Fluorine has seven valence electrons.
Total number of valence electrons for SF2 – 6 + 7*2 ( as there are two atoms of Fluorine, we will multiply the number by 2)
= 6 + 14
= 20 valence electrons
So, Sulphur Difluoride has a total of 20 valence electrons.
SF2 Lewis Structure
Lewis Structure is the pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. And now that we know the total valence electrons of SF2, we will start making the Lewis Dot Structure for this molecule.
Firstly, place the Sulphur atom in the centre as it is less electronegative than Fluorine. So it will be in the central position with both these Fluorine atoms on the terminal ends.
Fluorine atoms need one valence electron to complete its octet so it will share one valence electron of the Sulphur atom. So both the Fluorine atoms form a single bond with the Sulphur atom by sharing one valence electron of the Sulphur atom.
Each bond uses up two valence electrons so here four valence electrons are used from 20 valence electrons. As octets of Fluorine atoms are complete, put six valence electrons around each Fluorine atom. So a total of 16 valence electrons are used.
And Sulfur atom has four valence electrons that do not participate in bond formation and hence it is called lone pair or nonbonding pair of electrons. So in the Lewis Structure of SF2, there are single bonds between Sulphur and Fluorine atoms with two lone pairs of electrons on the central Sulphur atom.
SF2 Hybridization
To find out the Hybridization of this molecule, we will consider the two numbers of atoms and the total number of lone electron pairs bonded to the molecule. Here, if we look at the Sulphur atom, it is bonded with two atoms and has two electrons pairs. We get the final number 4, which corresponds to sp3 Hybridization. In fact, both Fluorine atoms are also sp3 hybridized. So, SF2 has sp3 Hybridization.
SF2 Molecular Geometry
The molecular geometry of the molecule depends on the Lewis structure and the arrangement of valence electrons in the structure. The sulfur atom has two bonding pairs of electrons and two nonbonding pairs of electrons that represent the VSEPR notion of AX2E2, which corresponds to an angular/non-linear or bent molecular geometry. So Sulfur Difluoride has a bent molecular geometry.
SF2 Shape
In the Lewis Structure of SF2, the central atom forms two bonds with two Fluorine atoms and has two lone pairs of electrons. The two lone pairs of electrons push the Fluorine atoms downwards due to the repulsive forces, and as a result, the shape of this molecule is bent.
Hence, SF2 is a bent-shaped molecule due to the presence of lone pairs on the Sulphur atom.
SF2 Bond Angles
The molecules with linear geometry have bond angles of 180 degrees but here as the shape of the molecule is bent due to the lone pairs on the Sulphur atom, both Fluorine atoms are pushed downwards, deviating the bond angle of F-S-F from 180 to 98 degrees.
Is SF2 polar or nonpolar?
To determine the polarity of any molecule, we check for the following factors:
- Presence of lone pairs
- The shape of the molecule
- The difference in electronegativities of atoms
- Net Dipole moment in the molecule
Sulfur Difluoride has a bent molecule geometry having two single bonds and two lone pairs of electrons. These lone pairs of electrons distort the shape of the molecule, and hence it is non-linear. As these lone pairs try to keep their repulsive forces minimal, they push down the Fluorine atoms.
Due to the presence of the lone pairs, there is symmetry in the molecule. And as a result, the charges will not be evenly distributed, increasing the chances of the polarity in the molecule.
When we compare Sulphur and Fluorine atoms’ electronegativities, the value of electronegativity of Sulphur is 2.58 and for Fluorine is 3.98. So here the difference of the electronegativities of both these atoms is much higher than 0.5, which makes the S-F bonds polar. And due to this vast difference in electronegativity, there will be a dipole moment between Sulphur and Fluorine atoms. The direction of the dipole moment will be from the Sulphur atom towards the Fluorine atom, as here Fluorine will try to pull the shared electrons to itself.
As this molecule is not linear, the dipole moments on both sides are not canceled out, resulting in the non-zero net dipole moment of the molecule. Hence, SF2 has poles in the molecule, where there are partial negative charges on the Fluorine atom and partial positive charges on the Sulfur atom which makes SF2 a polar molecule.
How Many Sulfur Valence Electrons
Concluding Remarks
To summarise this blog, we can say that,
- SF2 has a simple Lewis structure in which the Sulphur atom is in the centre forming single bonds with both the Fluorine atoms.
- There are two lone pairs of electrons on the Sulphur atom which makes the geometry of the molecule bent.
- The Sulphur atom has sp3 Hybridization, and the bond angle of F-S-F is 98 degrees.
- It is a polar molecule as there is a net dipole moment in the molecule.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies.
| Name of molecule | Sulfur Tetraflouride ( SF4) |
| No of Valence Electrons in the molecule | 34 |
| Hybridization of SF4 | sp3 hybridization |
| Bond Angles | 102 degrees and 173 degrees |
| Molecular Geometry of SF4 | Trigonal bipyramidal |
To understand this molecule’s properties, such as its reactivity, polarity, and more, one needs to know the SF4 Lewis structure first.
SF4 Molecular Geometry
It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the molecular formula of AX4E.
Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. As there is one lone pair on the central atom, it repels the bonding pair of electrons, which tweaks the shape a little bit and makes it appear like a see-saw. The electrons follow this pattern of arrangement following the VSEPR rule to minimize the repulsion forces between the lone pairs of electrons to maximize the molecule’s stability.
Hence, SF4 has a trigonal bipyramidal molecular geometry.
SF4 Lewis Structure
Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. The valence electrons that participate in forming bonds are called bonding pairs of electrons, whereas the electrons that do not participate or form any bonds are called nonbonding pairs of electrons or lone pairs.
And to draw the Lewis structure of SF4, we first need to know the total number of valence electrons in this molecule.
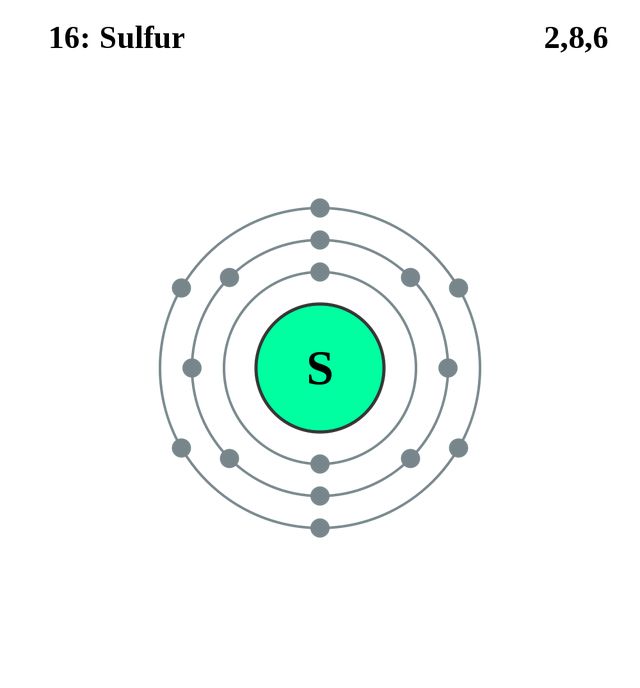
As one can probably see, there is one sulfur atom in this compound and four fluorine atoms. To know the total valence electrons of this compound, we need to know the valence electrons of both the atoms individually.
- Valence electrons of Sulfur: 6
- Valence electrons of Fluorine: 4* (7)
( as there are four fluorine atoms, we have to consider valence electrons of all atoms)
Total number of valence electrons in SF4 = number of valence electrons in sulfur + number of valence electrons in fluorine
= 6 + 28
= 34 valence electrons
Now that we know the total number of valence electrons, it would become easy for us to understand the bond formation between the atoms and the complete arrangement of the molecule too.
Sulfur will be the central atom in this molecule as it is the least electronegative, with four fluorine atoms forming bonds on the sides of this central atom. Every fluorine atom will form a bond with the central atom, which means there will be four bonds in the molecule structure using up four valence electrons of fluorine atoms and 4 electrons of the sulfur atom. So now, eight valence electrons are used, reducing the number of valence electrons from 34 to 24. All the fluorine atoms have six valence electrons, and the central atom has two valence electrons.
Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. Each fluorine atom will have three pairs of 6 valence electrons ( shown as dots) on the atom, along with one bond with sulfur. In contrast, the central atom will have two valence electrons and four bonds.
Hence, the central atom, sulfur, will have one lone pair of electrons and four bonding pairs of electrons in the Lewis structure of SF4. At the same time, each fluorine atom will have three lone pairs.
Sulfur Valence Electrons
Is SF4 polar?
Once we know the Lewis structure and molecular geometry of the given compound, it becomes easier to depict the molecule’s polarity. Here, one lone pair on the central sulfur atom and four bonding pairs of electrons leads to the asymmetric distribution of electrons on the central atom.
Also, as the shape of the molecule is like a see-saw, two fluorine atoms can cancel out each other’s dipole moment, but the rest two can’t due to the electrons’ arrangement. And as fluorine atoms are more electronegative than the sulfur atom, it results in uneven distribution of the charge. Hence the dipole moment is not canceled, which makes the molecule polar. So yes, SF4 is polar.
SF4 Hybridization
To know the hybridization of the SF4 molecule, let us first look at the regions of electron density for the central atom.
Sulfur has four bonding pairs of electrons and one lone pair, making its total number of regions for electron density 5. Hence the sulfur atom uses five hybridized orbitals, one 3s orbital, three 3p orbitals, and one 3d orbital. This arrangement of electrons around the atom and hybridized orbitals leads to the sp3d hybridization. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom.
Thus SF4 has sp3d hybridization.
SF4 Bond angles and shape
The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Fluorine atoms on the equatorial positions have the bond angles of 102 degrees, and the axial ones have 173 degrees, which are a little different than the trigonal bipyramidal molecular geometry leading to a see-saw shape.
The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms.
Concluding Remarks
To conclude all the properties we can say that,
Number Of Valence Electrons In Sulfur
- Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure.
- There are three lone pairs on each fluorine atom.
- It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry.
- SF4 has sp3d hybridization and is polar in nature.
